Abstract
Background: Sibling haplo donors can be broadly categorized as non-inherited maternal (NIMA) or paternal (NIPA) antigen mismatched based on the non-shared haplotype with the recipient. Older studies with non-PTCy prophylaxis suggested a benefit of NIMA-mismatched over NIPA-mismatched donors, but the same has not been studied with PTCy prophylaxis, especially in the context of increasing recognition of the impact of specific loci HLA-mismatches in haplo HCT, such as the B-leader, which is a leader peptide that is preferentially bound by HLA-E, the ligand for natural killer (NK) receptor NKG2.
Objective: To compare the outcomes of NIMA-vs NIPA-mismatched haplo sibling donors and specific loci HLA-mismatches in the setting of T-cell replete HCT with PTCy prophylaxis.
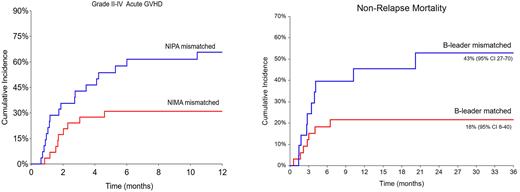
Results: We included 57 patients: 29 were NIMA-mismatched and 28 were NIPA-mismatched. Median age at HCT (31 yrs vs 37 yrs, p=0.1) and donor age (32 yrs vs 41 yrs, p=0.1) were similar in both groups. Most received RIC (72% vs 68%, p=0.5), BM graft (79% vs 82%, p=0.8), had HCT-CI >3 (55% vs 54%, p=0.9) and low/intermediate DRI (62% vs 54%, p=0.4) and were ABO matched (65% vs 71%, p=0.8) with the donor. There was a significantly higher proportion of female-donor to male-recipients in the NIMA- than NIPA-mismatched group (55% vs 21%, p=0.005). Specific HLA-mismatches were similar in both groups: B-leader (52% vs 32%, p=0.1), DRB1 (96% each), DQB1 (90% vs 86%, p=0.5), DPB1 (76% vs 79%, p=0.9). The median follow-up was 38 vs 13 months. There were no graft failures, and the median time to engraftment was similar (18 vs 19 days, p=0.3). The cumulative incidence of grade II-IV aGVHD was 31% vs 58%, p=0.04; with no differences in grade III-IV aGVHD (10% vs 11%, p=0.9), cGVHD (11% vs 13%, p=0.9); NRM (28% vs 33%, p=0.9), relapse (21% vs 11%, p=0.4), or OS (54% vs 55%, p=0.8) in NIMA-vs NIPA-mismatched, respectively.
In multivariate analysis, NIMA-mismatch (HR 0.1, 95% CI 0.02-0.6, p=0.01 in females) and HLA-A mismatch (HR 0.2, 95% CI 0.1-0.6, p=0.005) were associated with significantly lower risk of grade II-IV aGVHD, while age at HCT >50 yr was high risk for grade II-IV aGVHD (HR 2.7, 95% CI 1.1-6.9, p=0.04). No factor predicted grade III-IV aGVHD, cGVHD or relapse, likely related to a small number of events. B-leader mismatch was associated with high NRM (HR 3.8, 95% CI 1.04-13, p=0.04), poor PFS (HR 2.7, 95% CI 1.1-6.7, p=0.03), and a trend for lower OS (HR 2.4, 95% CI 0.9-6.3, p=0.06). High/very-high DRI was associated with high NRM (HR 4.4, 95% CI 1.5-13, p=0.008) and poor OS (HR 2.6, 95% CI 1-6.6, p=0.05). HCT-CI >3 (HR 2.9, 95% CI 0.9-8.8, p=0.05) was associated with poor OS. Neither NIMA-mismatch nor any other independent HLA-locus mismatches (except B-leader) had an impact on NRM, relapse, PFS, or OS.
Conclusion: Among sibling haplo donors, a B-leader matched donor should be prioritized whenever possible. When multiple B-leader matched donors are available, considering the sample size limitations, our data suggest that a donor mismatched at A-locus (lower aGVHD) and/or a NIMA-mismatched donor (lower aGVHD in females) could be selected.
Disclosures
Mehta:Syndax: Research Funding; Orca Bio: Research Funding. Alousi:Prolacta: Consultancy; Genetech: Consultancy; Mallinkrodt: Honoraria; Sanofi / Kadmon: Honoraria; Incyte: Honoraria, Research Funding. Kebriaei:Ziopharm: Research Funding; Amgen: Research Funding; Pfizer: Consultancy; Kite: Consultancy; Jazz: Consultancy. Nieto:Astra Zeneca: Research Funding; Affimed: Other: Scientific advisory Board, Research Funding; Secura Bio: Research Funding. Oran:AROG: Research Funding; ASTEX: Research Funding. Popat:Bayer: Research Funding; Incyte: Research Funding; Abbvie: Research Funding; Novartis: Research Funding; Iovance: Consultancy. Srour:Orca Bio: Research Funding. Rezvani:Navan Technologies: Other: Participates on the Scientific Advisory Board ; Bayer: Other: Participates on the Scientific Advisory Board ; GSK: Other: Participates on the Scientific Advisory Board ; Virogin Biotech: Other: Participates on the Scientific Advisory Board ; AvengeBio: Other: Participates on the Scientific Advisory Board ; GemoAb: Other: Participates on the Scientific Advisory Board ; Takeda: Patents & Royalties, Research Funding; Affimed: Research Funding; Caribou Biosciences: Other: Participates on the Scientific Advisory Board . Champlin:Kadmon: Consultancy; Bluebird: Other: Data Safety Monitoring Board; General Oncology: Other: Data Safety Monitoring Board; Omeros: Consultancy; Johnson &Johnson: Consultancy; Actinium: Consultancy; Cell Source Inc.: Research Funding. Shpall:adaptimmune: Consultancy; Takeda: Patents & Royalties; Bayer: Honoraria; Affimed: Other: License agreement; Fibroblasts and FibroBiologics: Consultancy; Navan: Consultancy; NY blood center: Consultancy; axio: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.